
Back in August 2020, Dr. Ronald B. Brown, PhD disrupted the academic world’s doomsday predictions about the COVID-19 pandemic when the journal Disaster Medicine and Public Health Preparedness published his first paper on the SARS-CoV-2 virus. As he told me in an interview:
The manuscript cites the smoking-gun, documented evidence showing that the public’s overreaction to the coronavirus pandemic was based on the worst miscalculation in the history of humanity, in my opinion.

On February 26, 2021, the peer-reviewed journal Medicina published another paper by Brown as part of a special issue, “Pandemic Outbreak of Coronavirus.” Brown’s paper, titled “Outcome reporting bias in COVID-19 vaccine clinical trials” is also listed in the U.S. National Library of Medicine of the National Institutes of Health.
In Brown’s first coronavirus paper, he showed how mistaking infection fatality rates for case fatality rates exaggerated the predicted lethality of the SAR-CoV-2 virus. In this second paper, he shows how relative risk reduction measures are being used to exaggerate the efficacy of the COVID-19 vaccines.
I’ve read the latest paper two-and-half times (but only claim to understand 90% of it). The overall conclusion, however, seems clear to me: The COVID-19 vaccine trials, in fact, only showed a negligible reduction in risk of acquiring a symptomatic SARS-CoV-2 infection; not the near perfect immunization the media is portraying.
As Dr. Brown writes in the paper’s conclusion:
Such examples of outcome reporting bias mislead and distort the public’s interpretation of COVID-19 mRNA vaccine efficacy and violate the ethical and legal obligations of informed consent.
The following is an informal interview I conducted with Dr. Brown, from his office in Kitchener-Waterloo, Ontario. It offers a layman’s interpretation of his findings and conclusions.
MANLEY: I’ve run into many people who refuse to even look at the vaccine trial data. They say they leave interpretation of the data to the “experts.” So, I’m glad we now have an expert like yourself to offer another interpretation of the data.
BROWN: But regardless of my expertise, I don’t have the power or license to tell people what to do. I don’t advise people. As a researcher, my goal is to present evidence so that people can choose to make more informed decisions about their health. I can explain the scientific evidence in layman’s terms, but I don’t think anyone, layman or expert, should take anything I “explain” on face value alone. Other experts could look at the same evidence and rightfully interpret it in an entirely different way, leading to an academic debate.
MANLEY: A debate? Aren’t those illegal? I guess not yet. But then, many people like to argue that there is no “right answer” because it is open for debate, and that we must rely on a consensus.
BROWN: As the evidence is presented from both sides during a debate, eventually the “truth” will emerge. By truth, I don’t mean merely a consensus. You can have 100% consensus that turns out to be 100% wrong, as in groupthink. Rather, I mean that the evidence is so clear that there is little point in arguing anymore… there is no longer any “reasonable doubt.”

MANLEY: Considering how little open debate there has been regarding not only the vaccine, but also COVID-19 itself, how close would you say we are to the truth?
BROWN: Today, we are nowhere near possessing knowledge that is beyond reasonable doubt concerning infectious viral diseases like COVID-19. Yet, as draconian public health mitigation measures are imposed on society with little proof of effectiveness, and much proof of collateral damage, there is little debate covered in the commercial media about public health issues. In my opinion, public health officials and politicians are under pressure to do something to protect the public, even if they have no idea what actually works. They see an open debate in the media as something that weakens their power and control.
There are other issues. The world copied China’s mitigation measures because China’s reported case rates are so low. But China’s rates are low because they use different case definitions than we do. If you want to instantly reduce cases of a disease, change the case definition. I have written about this in more detail in a new manuscript undergoing peer review. Also, we have a multitude of genomic sequencing technicians who are newly sequencing every common cold virus and variant they can find. Their findings are often translated immediately by public health officials, without sufficient vetting by epidemiologists who can put the information into proper context and prevent hysterical overreactions by public health officials and politicians.
Virology Cannot Answer Basic Questions
MANLEY: In many ways, we still don’t even understand how a virus functions, do we?
BROWN: What is a virus, where does it come from, what is its purpose, and what happens to it in the body? How pathogenic is it, and how infectious is it? Virology does not have the full answers to these basic questions, and yet, public health policy is predicated on assumptions about the nature of viruses that may prove to be the complete opposite of reality. I have spent the year reviewing the past and most recent virology literature, and I have come upon some astonishing evidence that could turn the whole infectious disease paradigm on its ear. That evidence will be presented in the near future in yet another manuscript currently under peer review.
MANLEY: Isn’t such exploration the basis of science? Wouldn’t such debate not only bring us closer to the truth, but also provide some sort of intellectual entertainment for the public?
BROWN: Yes, but a public health debate investigating these questions is being undermined by the official narrative dominating the commercial media. All other views are immediately dismissed in the commercial media as misinformation.
Modern Medicine Prone to Censorship
MANLEY: Would you agree that this type of censorship has been going on for probably as long as modern medicine has been around?
BROWN: Agreed, this is not unique to COVID-19. For example, I have tried to use the public media to report my novel evidence-based research findings about the cause of cancer, but with little success because my findings challenged the mainstream status quo (see Phosphate toxicity and tumorigenesis, 2018).
MANLEY: So how do we get the public more involved and interested in supporting open scientific debates?
BROWN: From open debates comes new knowledge, and new knowledge increases one’s power. The public must defend its right to access new knowledge, and the public should remain open-minded enough to consider all views. At the same time, one must remain skeptical and reject any explanation that is not backed up with sufficient evidence.
MANLEY: That’s where a lot of people have been trained to leave examining evidence to so-called “experts.”
BROWN: People can’t depend solely on the “approved” experts to tell them if the evidence is sufficient or not. We have so-called public health experts already telling us that now and look at the results. Experts from all sides must be given a fair hearing to present their case to the public and defend their case against the cases presented by other experts. It may be that pieces of evidence must be synthesized together from many sources to arrive at the final truth. That is the method I use to conduct my research. I look for pieces of evidence from a variety of research literature to synthesize together into a logical explanation or evidence-based theory (see Breakthrough knowledge synthesis in the Age of Google, 2020). If someone else presents additional evidence that refutes or proves my theory wrong, then everyone benefits and scientific knowledge advances.

MANLEY: Is that not where the public gets confused by their proud belief in “sound science” — relying on scientific theories rather than scientific evidence?
BROWN: Theories are just the starting point in the flow of scientific information, and the quality of a theory is related to the evidence upon which it rests. A good theory starts with a clean slate and inductively emerges out of the synthesis of reliable evidence. By contrast, evidence in a weak theory is cherry picked to support a predetermined conclusion or agenda, while ignoring contradicting or refuting evidence. But a weak theory doesn’t stand up to scrutiny.
In my vaccine manuscript, I included background information about Evidence-Based Medicine (EBM). Canada has been a major contributor to EBM through the work of David L. Sackett at McMaster University, who later worked at Oxford University. I added text to the manuscript citing Sackett’s research on clinical epidemiology. Sackett and Richard J. Cook, from the University of Waterloo, published clinical epidemiology tools to critically appraise the veracity and usefulness of clinical evidence in medical treatments and diagnosis. My manuscript attempts to carry on this great Canadian academic research tradition by applying these same clinical epidemiologic tools to a critical appraisal of mRNA vaccine clinical trials.
Why the COVID-19 Vaccine is Useless and Ineffective
MANLEY: Can you give us a layman’s explanation of your COVID-19 vaccine manuscript?
BROWN: The public and many health professionals are unaware of outcome reporting bias in COVID-19 vaccine clinical trials. Clinical trial outcomes reported by the Pfizer and Moderna vaccine manufacturers for their messenger RNA (mRNA) vaccines were reviewed and authorized for emergency use by an advisory committee of the Food and Drug Administration (FDA).
MANLEY: Do you know if the vaccines were actually approved or were they merely “authorized?” This is what the FDA did with the PCR tests, stating they were authorized for emergency use because they did not have an approved alternative. I was wondering if the same word game is being played here.
BROWN: It sounds like the same authorization for emergency use. The vaccines have not been officially approved, and the experimental trials are continuing. However, trial participants in the placebo group may choose to drop out to receive the vaccine, based on the too-good-to-be-true reported outcome of approximately 95% risk reductions in symptomatic SARS-CoV-2 infections.
MANLEY: Without an ongoing placebo group, would that mean, essentially, there is no long-term safety evaluation happening beyond the trial period?
BROWN: With more people dropping out, the statistical power of the study would weaken, although there are many thousands of people in the studies. More importantly, an ethical dilemma has surfaced to either encourage participants in the placebo group to drop out of a study and receive the vaccine benefits, or have those participants continue on with the placebo without the vaccine benefits. However, this dilemma assumes that the reported too-good-to-be-true efficacy of the mRNA vaccines is valid. My article uses clinical epidemiology tools to critically appraise the efficacy of the mRNA vaccine clinical trial outcomes. These tools are available online and may be used by anyone to verify the efficacy reported by the vaccine manufacturers, assuming that people can get their hands on reliable published data.
Also, since the article was published, follow up reports of observational studies have claimed that the vaccines are proving highly effective within the population. But the level of evidence in uncontrolled observational studies is inferior to that of clinical controlled trials, which is considered the gold standard of evidence. Observational studies may not compare results to control groups, and the studies don’t always adequately account for confounding factors, such as the deceleration of cases in the bell curve of seasonal influenza. Of course, people may protest that COVID-19 is much more lethal than seasonal influenza, but I exposed those biases in my first article. Furthermore, there are other biases in the reported high number of COVID-19 fatalities, which I critically appraise in my new manuscripts currently under peer review.
Relative Versus Absolute Risk Reduction
MANLEY: So exactly how much risk reduction are the manufacturers crediting their vaccine with?
BROWN: The reduced risk of COVID-19 infection reported by the manufacturers is approximately 95%, which is an accurate relative risk reduction measure. However, missing from the vaccine reports are absolute risk reduction measures which are much more clinically relevant to the reduced risk of COVID-19 infection. The absolute risk reduction of the vaccines in the present critical appraisal is approximately 1%, indicating practically no clinical efficacy or usefulness of the vaccines to reduce COVID-19 infection.
MANLEY: Essentially, then you are saying for all practical purposes, the vaccine is useless and ineffective?
BROWN: For applied clinical and public health interventions, yes, they appear to be almost completely ineffective. The members of the FDA advisory committee overlooked FDA guidelines to include absolute reduction measures when reporting clinical trial outcomes to the public, leading to outcome reporting bias in the FDA’s authorization of the mRNA vaccines.
MANLEY: Can you explain what is the difference between Relative Risk Reduction (RRR) and Absolute Risk Reduction (ARR)?
BROWN: Figure 2 in my article (shown below) sums up all the information you need to know as a layperson. The other calculations in the manuscript are intended for other researchers. You can calculate both relative risk reduction (RRR) and absolute risk reduction (ARR) from the same clinical trial data.
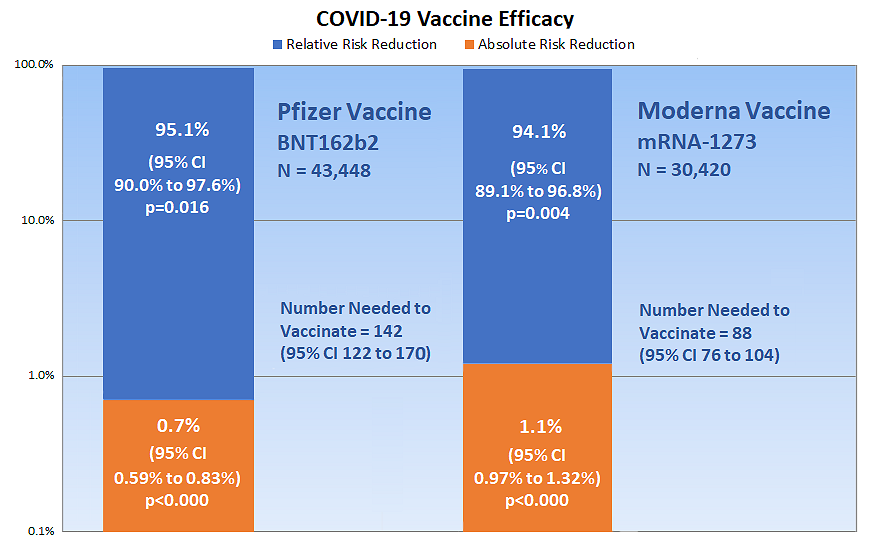
The Pfizer vaccine is represented by the column on the left of Figure 2, and the Moderna vaccine is on the right. The blue part of each column shows each vaccine’s relative risk reduction. This is the vaccine efficacy reported in the press.
MANLEY: So the Pfizer vaccine reduces the relative risk of SARS-CoV-2 infection by 95.1% and the Moderna vaccine reduces the risk by 94.1%, correct?
BROWN: Correct. So far, so good. However, what is not reported in the press, or in the clinical trial documents, is the orange portion of the columns showing the absolute risk reduction. This is only 0.7% (that’s seven-tenths of one percent) for the Pfizer vaccine, and 1.1% for the Moderna vaccine. These numbers are the most important numbers to consider when determining how much the vaccine will actually reduce your risk of infection. RRRs are intended for use in comparing an overall summary of one trial with other trials to determine which is more efficacious; RRRs are not intended for direct clinical and public health applications.
MANLEY: So, it appears as if they went with the relative risk reduction, because it looked more favourable?
BROWN: Yes, reporting relative risk outcomes, without absolute risk outcomes, has been a huge problem in research for decades. Notice that the ARR numbers are close to zero. The vaccines have almost no effect at all! In fact, the numbers are so low compared to the RRRs that I had to use a special percentage scale on the left of the figure that increases by ten times for each interval, otherwise the figure would be many times larger to span the enormous gap between the ARR levels and RRR levels.
MANLEY: Shouldn’t this be illegal? Or, at least, fall under the category of misleading advertising?
BROWN: The FDA guidelines say to report both RRRs and ARRs to the public, but the FDA advisory committees ignored the guidelines when they authorized the COVID-19 vaccines for emergency use, and they left out the ARRs. The New England Journal of Medicine also did not include ARRs when it published the clinical trial data for the vaccines. I agree with you that the people responsible for this misleading information should be held accountable. Check out the article’s reference to the roster members of the FDA advisory committee.
MANLEY: How do the COVID-19 risk ratios compare to influenza vaccines?
BROWN: That’s another bombshell in the article that people should be aware of. One of the peer reviewers suggested that I discuss other examples of outcome reporting bias involving relative risk measures in randomized clinical trials. My article shows that clinical trials of influenza vaccines have a 1.4% ARR compared to the usual 40% to 60% RRRs reported by the Centers for Disease Control and Prevention.
MANLEY: So, people are being led to believe that the COVID-19 vaccine(s) will all but eliminate their risks, when, the data suggests, it actually only makes a barely detectable difference?
BROWN: Correct. Some people may point out that 1% of a million vaccinated people are still 10,000 prevented symptomatic infections. Fair enough; then report a 1% reduction and see how many people are still interested in getting the vaccine. Furthermore, there is no reliable evidence that even a reported 1% reduction is valid. For example, normal saline solutions used in the placebo groups are associated with fevers and other symptoms common to coronavirus infections. The credibility of the entire enterprise is compromised.
Violating the Right to Informed Consent
Brown: This type of outcome reporting bias violates the public’s legal and ethical right to informed consent about the true efficacy of the vaccines. Regardless if you are provax or antivax or are undecided, you have a right to all the facts to inform your personal opinion and choice. Bottomline: you have before you smoking-gun evidence of a huge public health scandal — if the word ever gets out! This problem has been ongoing for decades and really took off when the pharmaceutical companies were granted permission to advertise directly to consumers in the 1980s. Think of all the systematic reviews of clinical trials that could be compromised by this type of clinical trial outcome reporting bias.

MANLEY: You were born in New York, but have lived in Ontario, Canada for the last 46 years. How open do you feel Canadians are to dissecting the claims being propagated around this COVID-19 vaccine?
BROWN: A Canadian friend told me that the truth is bad news. I thought to myself, “Think what you’re saying. You’re saying it is better to go along with what you are told, even though it is a lie.” Where I was raised (New York City), people are encouraged to speak out when they see something wrong. Apparently, Canadians aren’t encouraged to do that. Rocking the boat doesn’t fit in with the Canadian motto: Peace, Order, and Good Government (not great government, mind you, just good enough. Mustn’t set our expectations too high).
MANLEY: Yes, a Canadian businessman recently told me, “If you’re going to tell the truth, have one foot on your stirrup.” It is interesting that you, who are one of the few doctors in Canada to be speaking out, were actually born in the States. Anthony Fauci was also born in New York, was he not?
BROWN: Yes. And David L. Sackett, a founder of EBM, was also an American who immigrated to Canada. I came to Canada, in 1975, to teach music and perform as a professional musician. Fauci is from Brooklyn, and I was born in the Bronx, so he and I are part of a traditional NY rivalry going back to the Brooklyn Dodgers and the Bronx Bombers (Yankees) when I was growing up in the 50s.

Fauci and I obviously don’t see eye to eye. In a recent interview about the AZT clinical trials for AIDS, Fauci described what to do if the efficacy of a treatment “has not yet reached statistical significance.” Fauci’s quick-fix solution is that “the data needs to be further analyzed.” I don’t know of any other data analysis method that increases statistical significance as quickly as relative risk reduction measures. The public should be cautious of modern day snake-oil salesmen. Characters like that make a buck by filling people with fear and then selling a worthless quick-fix remedy to them. In my opinion, that’s exactly what’s happening in this pandemic.
MANLEY: Well, I’m glad you are on our side and have been able to have your work published in peer-reviewed journals.
BROWN: We live in a time of censorship and suppressed debate. Fear based on ignorance is the rule. The only way out is to publish the truth and science, have the public weigh the evidence, and let people make up their own minds. It’s a painfully slow process, and that’s frustrating, but I believe the truth will eventually win out. In the meantime, the only advice I can offer is for people to have patience. Have faith that when this is all over there will be a call for change and accountability.

Image by Dr. Brown, reminiscent of the snake-oil salesman from the American Wild West.
About Ronald B. Brown, PhD: He has authored over a dozen peer-reviewed articles in the U.S. National Library of Medicine of the National Institutes of Health; as well as a chapter on breakthrough knowledge synthesis in Contemporary Natural Philosophy and Philosophies. In addition to his epidemiologic research on infectious disease and vaccines during the COVID-19 pandemic, his current areas of research include prevention of cancer, cardiovascular disease, dementia, and other chronic diseases. You can read his paper, “Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials,” at the Multidisciplinary Digital Publishing Institute.

 About the Author: John C. A. Manley is the author of the full-length novel, Much Ado About Corona: Dystopian Love Story. He is currently working on the sequel, Brave New Normal, while living in Stratford Ontario, with his wife Nicole and son Jonah. You can
About the Author: John C. A. Manley is the author of the full-length novel, Much Ado About Corona: Dystopian Love Story. He is currently working on the sequel, Brave New Normal, while living in Stratford Ontario, with his wife Nicole and son Jonah. You can 